AN OVERVIEW OF MYOSTIM ED ERECTISTIM™ & ITS EFFICACY AGAINST ERECTILE DYSFUNCTION
BAILEY E. AUSTIN, M.Sc.
Since the 1990s, erectile dysfunction has been acknowledged as a prevalent condition, affecting 52% of the male population in the United States, according to the Massachusetts Male Aging Study1. Also known as ED, erectile dysfunction is a condition that prevents the ability to achieve an erection for an ongoing amount of time. ED not only affects men with stress and self-confidence issues, it is also a major contributor to interpersonal relationship problems with sexual partners. Pharmaceuticals are usually the first option men opt for when combatting ED. The problem with ED pharmaceuticals is that most of them target improving blood circulation TEMPORARILY, and that is not a sufficient approach to treating erectile dysfunction. PERMANENT blood circulation and penile muscle must be addressed when treating ED. MyoStim ED ErectiStim™’s revolutionary technology targets not only permanent blood circulation but muscle as well. In this white paper, we will further explore erectile dysfunction, MyoStim ED ErectiStim™ and technology behind this innovative product, clinical trial methodologies and clinical trial results.
UNDERSTANDING ERECTILE DYSFUNCTION
Erectile dysfunction is a condition that prevents the ability to achieve a sustainably adequate erection during sexual intercourse, for an ongoing amount of time2. ED can be caused by a variety of reasons, ranging from psychological to physiological. This condition can be major impact on the quality of life, including psychological stress, self- confidence issues, and interpersonal relationship problems with sexual partners6.
While there are numerous medications on the market that treat ED, some men do not respond to these medications. Other men may fear ED medication due to side effects and costs associated with medication1. Previously, an alternative to ED medication was not available, until now. MyoStim ED ErectiStim™ is the alternative solution to other ED therapies.
ABOUT MYOSTIM ED ERECTISTIM™
MyoStim ED ErectiStim™ is a bandage wrap that is applied to the affected penile tissue region. The micro-stimulator is located conveniently in the bandage wrap and is the culprit for the distribution of specific bioelectric signals to the affected penile tissue and penile nerves that regulate various protein expressions for stem cell homing, stem cell proliferation, stem cell differentiation, blood vessel forming and blood circulation improving, muscle function repair and DNA repair.
In very extreme cases, the bioelectric stimulation of MyoStim ED ErectiStim™ as described above is not a sufficient route of treatment for ED. The MS-15 Cocktail can be injected or infused from a refillable pump into the affected tissue region while simultaneously undergoing MyoStim ED ErectiStim™’s bioelectric stimulation treatment. The MS-15 Cocktail consists of adipose derived stem cells, growth factors, selected alkaloids, oxygenated nanoparticles, amniotic fluid, SVF, PRF, nutrient hydrogel and penile matrix.
MyoStim ED ErectiStim™’s ultimate goal is to provide those suffering from ED with a viable option of treatment that does not consist of shots and pills. Furthermore, our goal is to help men re-establish the erectile function they once had in their 30s. This therapy is to be administered at a 7-20 minute interval every other day for 12 weeks from the comfort of home! The next section will go into detail about the technology behind MyoStim ED ErectiStim™.

Current MyoStim ED ErectiStim portable device design

Electrode pad design

Future MyoStim ED ErectiStim portable device design
MYOSTIM ED ERECTISTIM™ TECHNOLOGY
MyoStim ED ErectiStim™’s technology is unique in that it not only targets a PERMANENT solution to blood circulation, but also repairs muscle associated with the affected penile tissue region with the application of bioelectrical stimulation therapy. Other ED therapies, especially pharmaceuticals, only achieve a TEMPORARY solution to blood circulation alone. Here at MyoStim ED, we strongly believe treating BOTH blood circulation and muscle is crucial to lasting erectile function. The main focus MyoStim ED ErectiStim™’s technology centers on the myostatin and follistatin balance.
Myostatin is a protein secreted in striated muscle tissue that stops the growth of muscle yet has never been reported present in smooth muscle tissue 5. With this being said, Dr. Kovanecz, a leading research professor with UCLA’s Department of Urology, conducted a study in 2017 that found myostatin present in the smooth muscle cells of rat penile tissue4. Dr. Kovanecz and his team not only discovered myostatin in the penile corpora, but also in vascular regions of arteries, veins, and the aorta of the rats4. Therefore, it should not come as a shock that research has found increased levels of myostatin levels linked to a number of muscle disorders, especially those associated with ED. Blocking the myostatin pathway is the key to solving this problem.
MyoStim ED ErectiStim™’s bioelectric stimulating therapy plays a major role of controlling the release of follistatin, a glycoprotein, that is not only a potent antagonist of myostatin that works to block myostatin but also increases penile muscle mass and strength 5,7. Originally derived from the ovarian follicular fluid, follistatin stops the sectrition of follicle stimulating hormone (FSH) from the pituitary gland 7.
In addition to releasing follistatin, MyoStim ED ErectiStim™ is designed to release these regenerative proteins, a combination that aids in improved blood circulation and muscle repair:
– SDF-1 for stem cell homing
– IGF-1 for DNA repair
– eNOS for dilating blood vessels
– SDFI for improved blood circulation
– EGF, HGF, PDGF, VEGF, SDF, HIF1A, and CXCL5 for growing new blood vessels
– Tropoelastin for improving elasticity
– Activin A for improving nerve connections
INITIAL CLINICAL TRIAL METHODOLOGIES & RESULTS
The initial clinical trial was conducted at the Federal University of Health Sciences in Brazil, with Dr. Cristiane Carboni as principal investigator. The randomized clinical trial consisted of twenty-two ED patients. Patients were randomly placed in to two groups: intervention and control 1. The intervention group endured FES therapy (50 Hz/500 μs) during a four-week time interval, involving twice weekly 15-minute therapy sessions, ensuring that the stimulant intensity was lower than the motor threshold 1. The control group endured placebo FES, mimicking the same routine as the intervention group as described. The International Index of Erectile Function (IIEF-5) and Erection Hardness Score (EHS) were the validated tools used to evaluate erectile function, along with application before and after treatment, and quality of life, by the WHOQOL questionnaire 1.
Two statistically substantial enhancements were noted in the intervention group. For starters, the IIEF-5 and EHS scores reflect a substantial contrast in the erectile function between the intervention group pre- and post-treatment with the control group1. Next, there was a substantial contrast between the pre-treatment and post- treatment time points in the intervention group1. The environment realm reflected no difference for the intervention group in the WHOQOL-BREF questionnaire; the control group reflected no differences in any questionnaires1. The initial study focuses solely on the VEGF signal, one of the regenerative proteins responsible for the growth of new blood vessels. What was discovered was that the VEGF creates leaky blood vessels that retreat and do not last1.
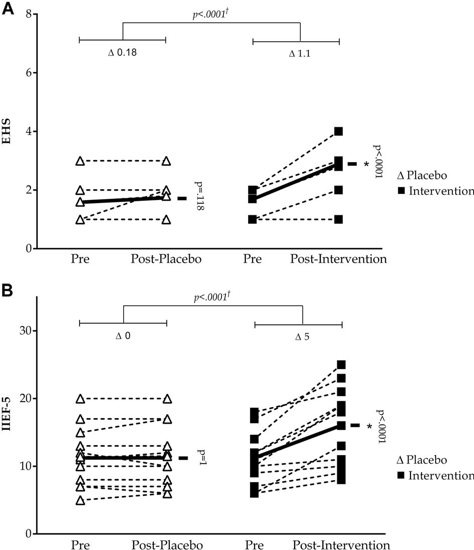
Individual changes in the EHS score (a) and IIEF-5 score (b)
A follow up clinical trial is currently being conducted by Dr. Carboni and team, but in this new trial there are 30 patients, divided into three groups. Group I is a control group identical to the first trial, Group II is a group identical to the intervention group in the first trial (with a focus solely on VEGF), and Group III receives the same therapy as the first groups (with a focus on not only VEGF but eNOS, SDF, IGF, and follistatin as well).
In summary, MyoStim ED ErectiStim™ has the potential to provide these product design benefits:
• High precision bench top stimulator or small hand held and easy to use device options
• Simple to administer pre-set stimulation treatment delivered by external penal self-adhesive skin electrodes (i.e. reusable electrodes)
• Comfortable during treatment with high user adherence to treatment regime
• Fifteen minutes treatment every other day for four to eight weeks, with improvements observed at the four-week mark
• For long term maintenance, fifteen minute treatments per month after the initial four to eight weeks
• Class 2a Medical Device (CE0124)
MyoStim ED ErectiStim™ has the potential to provide these technology benefits, when pharmaceuticals cannot deliver:
• Therapeutic success in excess of 85%
• A bioelectric stimulator apparatus that can control the release of stem cell homing, new mature (non-leaking) blood vessel growth and muscle repair
• Biolelectric stimulation control of SDF-1 for stem cell homing
• Bioelectric stimulation signaling for VEGF, PDGF, EGF, HGF, HIF1a, and CXCL5 for mature new blood vessel formation
• Bioelectric stimulation signaling for eNOS for dilation.
• Achieving a follistatin myostatin balance through bioelectric stimulation therapy
• Bioelectric stimulation of tropolelastin to improve elasticity of stimulated tissues when needed
• A potentially novel therapeutic option for ED
• Safety and efficacy demonstrated in small pilot study but not yet in statically significant double blinded, randomized, placebo controlled study.
MyoStim ED ErectiStim™ is ranked superior in comparison to other treatment therapies of ED because it not only establishes PERMANENT blood circulation, but also repairs muscle damage associated with ED. Its ergonomical bandage wrap design, combined with the ease of administrating the therapy from the comfort of home, makes MyoStim ED ErectiStim™ the ideal therapy when treating erectile dysfunction. MyoStim ED ErectiStim™ is an innovative and revolutionary ED therapy that will not only change the intimate lives of all that suffer from erectile dysfunction, but rid the need for complicated shot and pill ED therapy regimens.
REFERENCES
1. Carboni C, Fornari A, Bragante K, Averbeck M, Vianna da Rosa E, Plentz R. An initial study on the effect of functional electrical stimulation in erectile dysfunction: a randomized controlled trial. International Journal of Impotence Research. 2018; 30:97-101.
2. Heruti, Rafi & Shochat, Tzipi & Tekes-Manova, Dorit & Ashkenazi, Itshak & Justo, Dan. (2004). Prevalence of Erectile Dysfunction Among Young Adults: Results of a Large-scale Survey. The Journal of Sexual Medicine. 1. 284-91. 10.1111/j.1743-6109.04041.x.
3. Kovanecz, I., Masouminia, M., Gelfand, R.M., Vernet, D.A., Rajfer, J., & Gonzalez-Cadavid, N.F. (2017). Myostatin, a profibrotic factor and the main inhibitor of striated muscle mass, is present in the penile and vascular smooth muscle. International Journal of Impotence Research, 29, 194-201.
4. Rodino-Klapac, L. R., Haidet, A. M., Kota, J., Handy, C., Kaspar, B. K., & Mendell, J. R. (2009). Inhibition of myostatin with emphasis on follistatin as a therapy for muscle disease. Muscle & nerve, 39(3), 283-96.
5. Wood, H. (2018 June). Erectile Dysfunction. Retrieved from
http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/endocrinology/erectile-dysfunction/
6. Zhu, J., Li, Y., Lu, A., Gharaibeh, B., Ma, J., Kobayashi, T., Quintero, A. J., … Huard, J. (2011). Follistatin improves skeletal muscle healing after injury and disease through an interaction with muscle regeneration, angiogenesis, and fibrosis. The American journal of pathology, 179(2), 915-30.
Warning Caution and Disclaimer: Not available for sale in the USA. For investigational use only outside of the USA only at this time with proper regulatory clearances and training. The pre-clinical and pilot study results that may be shared are not conclusive in proving either safety or efficacy. Statistically significant controlled double blinded placebo controlled studies have not been completed yet.
